Background
For several years leading up to 2022, I had experienced a gradual decline in the number of primary crystals in my glazes. It wasn’t a problem initially, small changes in crystal forming tendency can be compensated for by adjustment of the the glaze composition. There is a limit to this and a point was reached with several of my glazes where further adjustment would have changed their character. One possibility I feared was a gradual deterioration of the temperature sensing thermocouples, or the calibration of the amplification electronics. A careful study of the firing records showed a different pattern. It looked as though the reduction in crystals was caused by packing the kiln with more ware. I have been steadily diversifying my range of work and that has allowed me to use much more of the kiln volume. I now pack firings very tightly and this makes the kiln cool more slowly from the top temperature to the crystal growing temperature, a part of the firing cycle that I do not currently control. This motivated me to find a calculation that would quantify the effect so that I could take it into account. Here I present the initial work, the development of a procedure for calculating the ‘heat-work’ of a firing from the time-temperature curve.
Introduction
Precise control of the glaze firing is essential for producing consistent results with pottery and especially with crystalline glazes. Modern kiln controllers are able to control temperature reliably to a schedule. The question addressed here is what kind of temperature schedule works best under a wide range of conditions.
Pyrometric cones are a valuable tool in assessing what it termed ‘heat-work’, that is, the combined effects of both time and temperature on the contents of the kiln. A complementary approach is to model ‘heat-work’ mathematically in order to give a similar, but more convenient, result to pyrometric cones. The function that does this is known as a ‘master sintering curve’ [1]. It has the property that it correctly accounts for the entire temperature – time profile of a firing in a similar way to a pyrometric cone. All firings that produce the same cone, or heat-work, have the same value of the function. This is why it is called a ‘master curve’ – it correctly maps the entire range of possible firing schedules into a single value.
The heat work in a firing depends not only on the maximum temperature reached but also on how rapidly the kiln heats up, how long a soak there is at the maximum temperature, and how rapid is the subsequent cooling.
The master sintering function presented here quantifies these effects allowing their relative contributions to be clearly seen. It answers the questions of just how important the rate of temperature rise is together with the relative importance of temperature control during cooling.
The master sintering function
This master sintering function is based on the simplifying assumption that the densification kinetics of a porcelain body are controlled by a dominant diffusion process. A number of investigators [1,2] have successfully used a modified Arrhenius function to model this rate. This leads to the form of the master sintering function given in equation (1).
(1) ![]()

The function directly represents the amount of heat work performed.
Here the activation energy, Q depends on the type of body being used. In simple terms, it represents what might be called the ‘fusibility’ of the body. It will be different for an earthenware body, a cone 6 porcelain and a cone 10 porcelain.
This study is focused on a cone 10 body. An activation energy of 805kJmol-1 gives results that are in very good agreement with observations of actual firing results. In addition, this value gives the same value of Θ for Orton cone 10 for all of the heating rates for which the temperature endpoint is specified (by Orton). The figure for activation energy is somewhat higher than values stated in the literature for cone 10 porcelain bodies [3.4]. More work is needed to refine the figure. A different type of porcelain, for example one maturing at cone 6, would be modelled using a different activation energy.
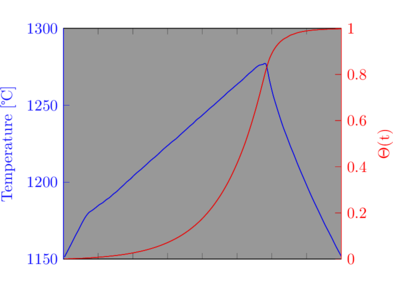
An example taken from one of my crystalline firings illustrates how the function can be used. The temperature profile shown in figure 1 is typical of my firings: a linear ramp up to the top temperature, a short soak followed by uncontrolled cooling. The value of ![]() has been normalised in this case so that a value of 1.0 represents the final amount of heat work for the firing. The calculation shows several important features. Below 1150
has been normalised in this case so that a value of 1.0 represents the final amount of heat work for the firing. The calculation shows several important features. Below 1150![]() the amount of heat work is negligible. As the top temperature is reached and the short soak begun,
the amount of heat work is negligible. As the top temperature is reached and the short soak begun, ![]() has increased to 0.65 and by the end of the soak is 0.82. The uncontrolled cooling phase is responsible for the remaining heat work, 18% of the total.
has increased to 0.65 and by the end of the soak is 0.82. The uncontrolled cooling phase is responsible for the remaining heat work, 18% of the total.
 Calculation details
Calculation details
![]() is calculated by numerical integration of the kiln temperature-time profile (i.e. the function
is calculated by numerical integration of the kiln temperature-time profile (i.e. the function ![]() (with the temperature expressed on the absolute Kelvin scale). The method of Romberg has been used for the examples given here. An excellent discussion of this method and a sample implementation are given by William Press et al. [4].
(with the temperature expressed on the absolute Kelvin scale). The method of Romberg has been used for the examples given here. An excellent discussion of this method and a sample implementation are given by William Press et al. [4].
References
[1] Su, H. and Johnson, D.L; ‘Master Sintering Curve: A Practical Approach so Sintering’; Journal of the American Ceramic Society, 1996, 79, (12) 3211-3217.
[2] Mao-Hua Teng, Yi-Chun Lai and Ying-Tien Chen; ‘A computer program of master sintering curve model to accurately predict sintering result’; Western Pacific Earth Sciences vol.2, no.2, p.171-180, May, 2002.
[3] Çağatay Koca, Nuray Karakus, Nil Toplan and H. Özkan Toplan; ‘Use of borosilicate glass waste as a fluxing agent in porcelain bodies’; January 2012 Journal of Ceramic Processing Research 13(6):693-698.
[4] W.H. Press et al.;’Numerical Recipes in C: The Art of Scientific Computing, 2nd Edition’; Cambridge University Press, 1982.
